EBV-induced B cell immortalization and latency
Epstein-Barr virus (EBV), or Human Herpesvirus 4 (HHV4), is a gamma-herpesvirus that infects more than 90% of the world human adult population. After primary infection that is generally asymptomatic, EBV establishes lifelong persistence of latent viruses in B lymphocytes and in epithelial cells of the oropharynx that are the predilected, but not the exclusive, cellular targets. In these reservoirs, latent viruses express different latency expression programs that induce immortalization of infected cells. EBV-induced cellular proliferation is tightly controlled by the immune response with up to 1% of the host cytotoxic T cells targeting EBV infected cells, keeping the proportion of EBV-infected B lymphocytes around 1 infected cell/million B lymphocytes. Reactivations of viral lytic replication with virions production and cell lysis occur sporadically and are favoured by immunosuppression and B-cell stimulation.
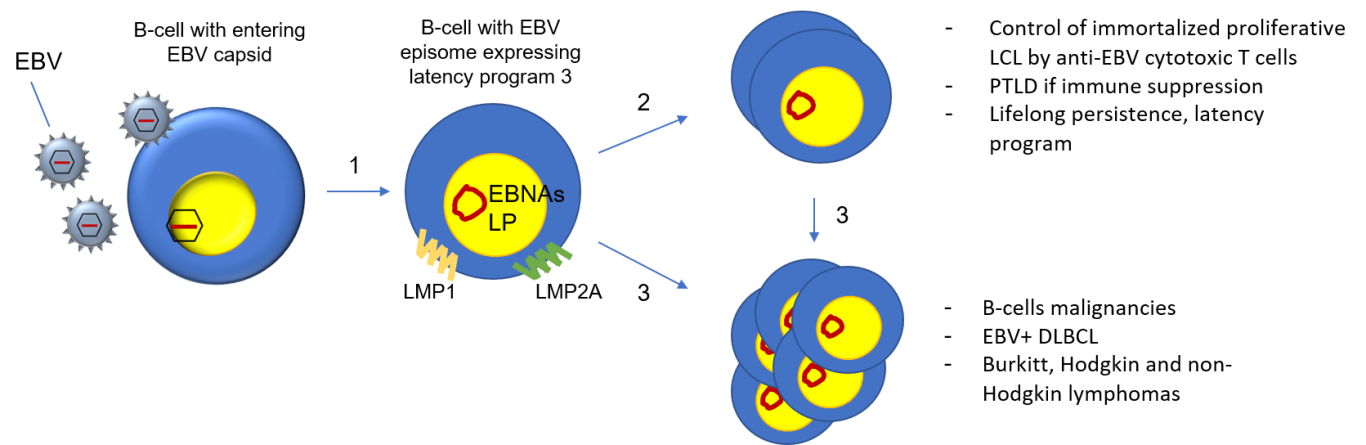
- In B cells, EBV infection leads to the formation of stable EBV episome (circular dsDNA) expressing the latency program 3 (EBNAs, LP, and LMP1-2A) that induces the immortalization and proliferation of B-cells.
- The EBV-infected B cells continuously expand forming polyclonal cell lines known as lymphoblastoid cell lines (LCL). This expandable reservoir of immortalized EBV+ B-cells is controlled by the immune cytotoxic response. Immune suppression favours the development of PTLD.
- Environmental and genetic factors, chronic B-cells stimulation, and immune-suppression favour the transformation of already immortalized and proliferating B-cells, leading to the development of lymphomas and related malignancies.
EBV associated disorders
EBV is a major pathogen associated with a wide range of disorders, including malignancies and autoimmune diseases.
Malignancies
EBV is associated with more than 250 000 new malignancies worldwide per year and was the first recognized human oncogenic virus. Malignancies to which EBV is linked at various frequencies are Burkitt and Hodgkin lymphomas, non-Hodgkin lymphomas, …., as well as T cell lymphomas and T/NK lymphomas. EBV is found in almost 100% of naso-pharyngeal carcinomas,
Post-transplant lymphoproliferative disorders (PTLD) are observed in 2-20% of patients after organs or bone marrow transplantation and is one of the worst complications of organ transplantation. PTLD occurring during the first-year post-transplantation are associated with EBV in >90% of cases. Lymphoproliferation concerns typically EBV infected B-lymphocytes following de novo infection of naïve B cells or after latent virus reactivation. EBV-positive PTLD histopathological classification ranges from infectious mononucleosis like syndrome, to polymorphic PTLD, and to B-cell neoplasms (Burkitt, DLBCL, others).
Multiple sclerosis
EBV was recently recognized as a necessary but not sufficient prerequisite in the pathogenesis of multiple sclerosis (MS). MS is the most frequent demyelinating disease leading to disruption of the neuronal signal transmission with a wide range of debilitating symptoms.
EBV primary infection precedes MS onset for almost 100% of patients and EBV-positive B and plasma cells are fond in the central nervous system of these patients with an oligoclonal secretion of gamma globulins. Strong structural homology exists between several epitopes in the EBV EBNA1 protein and myelin suggesting an auto-immune mediated mechanism in axon demyelination.
Therapeutic trials that induce nonspecific B cell depletion (CD20 depletion, cladribine) have shown a decrease in MS activity. Cladribine received FDA and EMA approval.
